Pyrimidine
| Pyrimidine | |
|---|---|
 |
|
|
Pyrimidine
|
|
|
Other names
1,3-Diazine, m-Diazine
|
|
| Identifiers | |
| CAS number | 289-95-2 |
| PubChem | 9260 |
| MeSH | pyrimidine |
|
SMILES
C1=CN=CN=C1
|
|
| Properties | |
| Molecular formula | C4H4N2 |
| Molar mass | 80.088 |
| Melting point |
20–22 °C |
| Boiling point |
123–124 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring.[1] It is isomeric with two other forms of diazine.
Contents |
Nucleotides
Three nucleobases found in nucleic acids, cytosine (C), thymine (T), and uracil (U), are pyrimidine derivatives:
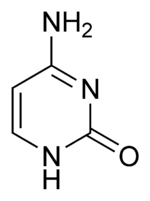
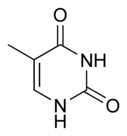
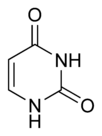
In DNA and RNA, these bases form hydrogen bonds with their complementary purines. Thus, the purines adenine (A) and guanine (G) pair up with the pyrimidines thymine (T) and cytosine (C), respectively.
In RNA, the complement of A is U instead of T and the pairs that form are adenine:uracil and guanine:cytosine.
It is very rare, however, that thymine occur in RNA or uracil in DNA. Other than the three major pyrimidine bases presented, some minor pyrimidine bases can occur in nucleic acids as well. These minor pyrimidines are usually methylated versions of major ones and are postulated to have regulatory functions. [2]
These hydrogen bonding modes are for classical Watson-Crick base pairing. Other hydrogen bonding modes ("wobble pairings") are available in both DNA and RNA, although the additional 2'-hydroxyl group of RNA expands the configurations, through which RNA can form hydrogen bonds.
Chemical properties
A pyrimidine has many properties in common with pyridine, as the number of nitrogen atoms in the ring increases the ring pi electrons become less energetic and electrophilic aromatic substitution gets more difficult while nucleophilic aromatic substitution gets easier. An example of the last reaction type is the displacement of the amino group in 2-aminopyrimidine by chlorine[3] and its reverse.[4] Reduction in resonance stabilization of pyrimidines may lead to addition and ring cleavage reactions rather than substitutions. One such manifestation is observed in the Dimroth rearrangement.
Compared to pyridine, N-alkylation and N-oxidation is more difficult, and pyrimidines are also less basic: The pKa value for protonated pyrimidine is 1.23 compared to 5.30 for pyridine.
Pyrimidine also is found in meteorites, although scientists still do not know its origin. Pyrimidine also photolytically decomposes into Uracil under UV light.[5]
Organic synthesis
Pyrimidines can also be prepared within the laboratory by organic synthesis. One method is the classic Biginelli reaction. Many other methods rely on condensation of carbonyls with amines for instance the synthesis of 2-Thio-6-methyluracil from thiourea and ethyl acetoacetate [6] or the synthesis of 4-methylpyrimidine with 4,4-dimethoxy-2-butanone and formamide [7].
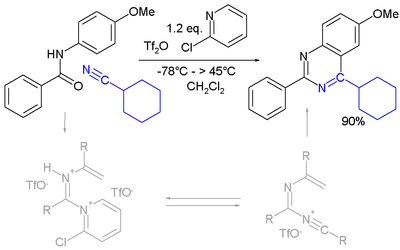
A novel method is by reaction of certain amides with carbonitriles under electrophilic activation of the amide with 2-chloro-pyridine and trifluoromethanesulfonic anhydride [8]:
See also
- Pyrimidine biosynthesis
- Pyrazine, an analog with the nitrogen atoms in positions 1 and 4
- Pyridazine, an analog with the nitrogen atoms in positions 1 and 2
- Simple aromatic rings
- ANRORC mechanism
References
- ↑ Heterocyclic Chemistry (3rd Edition) Thomas. L. Gilchrist ISBN 0-582-27843-0
- ↑ Nelson David L. and Michael M. Cox. Principles of Biochemstry, ed. 5. W.H. Freeman and Company (2008) p. 272-74.
- ↑ Organic Syntheses, Coll. Vol. 4, p.182 (1963); Vol. 35, p.34 (1955) Link
- ↑ Organic Syntheses, Coll. Vol. 4, p.336 (1963); Vol. 35, p.58 (1955) Link
- ↑ Nuevo M, Milam SN, Sandford SA, Elsila JE, Dworkin JP (2009). "Formation of uracil from the ultraviolet photo-irradiation of pyrimidine in pure H2O ices.". Astrobiology 9 (7): 683–95. PMID 19778279.
- ↑ Organic Syntheses, Coll. Vol. 4, p.638 (1963); Vol. 35, p.80 (1955) Link
- ↑ Organic Syntheses, Coll. Vol. 5, p.794 (1973); Vol. 43, p.77 (1963) Link
- ↑ Single-Step Synthesis of Pyrimidine Derivatives Mohammad Movassaghi and Matthew D. Hill J. Am. Chem. Soc.; 2006; 128(44) pp 14254 - 14255; (Communication) doi:10.1021/ja066405m
|
|||||||||||||||||||||||||||